Abstract
Background
Long-term survival and late mortality risk compared to general population for patients (pts) who underwent an allogeneic hematopoietic cell transplant (HCT) is unknown. We analyzed long-term outcomes of 2-year (yr) HCT survivors with acute lymphoblastic leukemia (ALL).
Methods
Adult pts with ALL who were alive and relapse-free at 2 yrs after first HCT from 2005-2012 were included. We excluded patients who had a cord blood transplant and ex vivo T cell depletion (TCD). Relative survival analysis was used to estimate HCT-related crude mortality taking into account for background population mortality rates of the general population, matched for age, sex, and country in the year of HCT (www.mortality.org).
Results
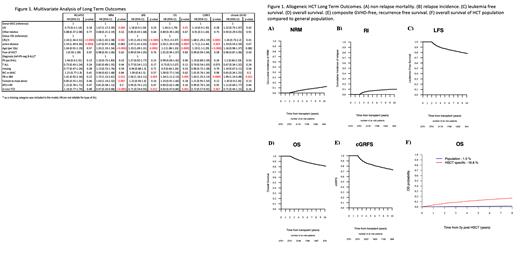
A total of 2701 pts were included with a median follow up interval of 99 months and median age of 34 (range 18 - 73.5) yrs. The majority (78.6%) of pts were in 1 st complete remission (CR1) with undetectable MRD (68.3%). There were similar numbers of matched sibling donor (MSD) (43.7%) and unrelated donor transplants (MUD) (53.2%). Most pts received myeloablative conditioning (MAC) (86.5%) and peripheral blood (PB) grafts (75.7%) without in vivo TCD (55.7%).
The 10-yr probability for overall survival (OS) and leukemia-free survival (LFS) was 81.3% and 78.2%, respectively. Cumulative incidence of disease relapse and non-relapse mortality (NRM) at 10 years was 9.9% and 11.9%, respectively. The probability of chronic GVHD-relapse-free survival (cGRFS) at 10-yrs was 73.3% (Figure 1). Relapsed ALL and chronic GVHD were common causes of late mortality accounting for 33.9% and 29% of reported deaths, respectively, followed by infection and secondary malignancy. For patients transplanted in countries with available mortality data (92% of patients in our cohort), the probability of dying from another cause is negligible at 1.5% compared to the probability of dying from HCT (16.8%) 10 years after HCT (Figure 1F).
Conclusions
In a large registry-based study, we showed excellent long-term survival of 81.3% at 10-yr among the 2-yr survivors of HCT for ALL. There was no difference in long term outcomes with respect to conditioning intensity, but utilization of BM graft and in vivo TCD resulted in lower NRM, and better OS. Long-term mortality risk among HCT survivors remains significantly higher than expected for the general age-matched population.
Labopin: Jazz Pharmaceuticals: Honoraria. Schroeder: Celgene: Honoraria, Other: Travel support, Research Funding. Bethge: Miltenyi Biotec: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Kite-Gilead: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau. Nicholson: Pfizer: Consultancy; BMS/Celgene: Consultancy; Kite, a Gilead Company: Other: Conference fees, Speakers Bureau; Novartis: Consultancy, Other: Conference fees. Giebel: Janssen: Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau. Peric: Therakos, Servier, MSD, Astellas, Novartis, Abbvie, Pfizer: Honoraria. Dholaria: Janssen: Research Funding; Pfizer: Research Funding; Takeda: Research Funding; Jazz: Speakers Bureau; MEI: Research Funding; Angiocrine: Research Funding; Poseida: Research Funding; Celgene: Speakers Bureau. Mohty: Sanofi: Honoraria, Research Funding; Pfizer: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Jazz: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria; Celgene: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria; Astellas: Honoraria; Amgen: Honoraria; Adaptive Biotechnologies: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal